Acids, Bases and Salts
Chemical properties ke hisaab se compounds 3 types ke hote hain:
- Acids (Khatte)
- Bases (Kadve/Kasaile)
- Salts (Namak jaise)
Download Free Acids, Bases and Salts Class 10 Handwritten Notes PDF
Indicators (Check karne ka tareeka)
Indicator ek aisi dye hoti hai jo Acid ya Base mein daalne par apna colour badal leti hai.
- Common Indicators:
- Litmus: Acid blue litmus ko Red kar deta hai. Base red litmus ko Blue kar deta hai. (Trick: Base = Blue).
- Methyl Orange: Acid mein Red, Base mein Yellow.
- Phenolphthalein: Acid mein Colourless (paani jaisa), Base mein Pink.
- Natural Indicators:
- Litmus: Yeh ‘Lichen’ naam ke plant se banta hai.
- Turmeric (Haldi): Base mein Red ho jati hai (isliye sabun lagne par kapde ka daag laal ho jata hai).
- Red Cabbage: Acid mein Red rehta hai, par Base mein Green ho jata hai.
3. Olfactory Indicators (Naak se pehchano ):
Inki khushbu (smell) badal jati hai.
- Onion & Vanilla: Acid mein inki smell nahi jati, par Base mein inki smell gayab ho jati hai.
Acids (Details)
Acids taste mein sour (khatte) hote hain.
- Organic Acids: Jo plants aur animals mein natural milte hain. Ye khane mein safe hote hain.
- Vinegar ➔ Acetic acid
- Lemon ➔ Citric acid
- Curd ➔ Lactic acid
- Ant sting (Cheenti ka kaatna) ➔ Formic acid
- Lactic acid-→ sour milk (or curd)
- Tartaric acid → tamarind & unripe grape
Mineral Acids: Jo zameen ke minerals se bante hain (Man-made). Ye bahut khatarnaak hote hain.
- Eg: HCl, H2SO4 (Sulphuric acid).
Dilution (Acid mein paani milana)
Dhyan rakho! Kabhi bhi Acid mein paani mat daalna (blast ho sakta hai). Hamesha Paani mein Acid dheere-dheere daalna chahiye aur hilate rehna chahiye.
Acid ki Properties (Reactions)
- Electricity: Acid ka solution electricity conduct karta hai.
- Metal ke saath:
Metal + Acid ➔ Salt + Hydrogen Gas
Zn(s) + H2SO4(aq) ➔ ZnSO4 + H2 zinc (sulphuric acid) zincsulphate
Note: Isliye khatti cheezein (dahi, nimbu) metal ke bartan mein nahi rakhni chahiye, kyunki woh zeherila (poisonous) reaction kar sakti hain.
- Jab Acids react krta hai metal carbonates to carbon dioxide gas bnti hai
Metal carbonate + Acid → salt + carbon dioxide + water
Na2CO3(s) + 2HCl → NaCl + CO2 + H2O sodium carbonate
metal Hydrogen carbonate + Acid → salt + carbon dioxide + water
NaHCO3 + HCl→2NaCl(aq) +CO2(g) +H2O(l)
When carbon dioxide gas is passed through lime water, the lime water tums milky due to the formation of a white precipitate of calcium carbonate.
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
calcium Hydroxide calcium carbonate
(Limewater) (white ppt.)
6. Acids react with bases to form salt and water.
Acid + Base → salt + water
Note:- The reaction between an acid and a base to form salt and water is called a neutralisation reaction.
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
- Jab acid metal oxide ke saath reaction, Karta hai Toh salt or water Banta hai.
Metal oxide + Acid → salt + water
CuO(s) + 2HCl(aq) → CuCl2(aq) + H2O
(Copper oxide) (copper chloride)
Black Blue-green
What do all acids have in Common
Sabhi acids ki ek common baat hoti hai ki jab ye paani (water) me ghole jaate hain to Hydrogen ions (H⁺) produce karte hain।
- Glucose aur alcohol ka aqueous solution acidic nature nahi dikhata, kyunki inme jo hydrogen hota hai wo paani me ghulne par H⁺ ions ke roop me alag nahi hota।
Note: Sabhi acids me hydrogen hota hai, lekin har hydrogen-wala compound acid nahi hota।
Strong Acid
Jo acid paani me completely ionise ho jata hai aur zyada matra me H⁺ ions deta hai, use strong acid kehte hain।
Example:
- Sulphuric acid (H2SO4)
- Nitric acid (HNO3)
Weak Acid
Jo acid paani me partially or thoda sa ionise hota hai aur kam matra me H⁺ ions deta hai, use weak acid kehte hain।
Example:
- Acetic acid
- Carbonic acid
Uses of Mineral Acids in Industry
- Sulphuric acid ka use fertilizers, paints, dyes, detergents or car batteries banane me hota hai
- Nitric acid ka use fertilizers, explosives, dyes aur plastics banane me hota hai।
- Hydrochloric acid ka use steel objects par jammi hui oxide layer ko hatane ke liye hota hai।
Bases
Bases wo chemical substances hote hain jinka taste kadwa (bitter) hota hai।
- Jo base paani me soluble hota hai use alkali kehte hain।
Sabhi Bases ki Common Baat
Sabhi bases ki ek common property hoti hai ki jab ye paani me ghole jaate hain to Hydroxide ions (OH⁻ ions) produce karte hain।
Strong Base
Jo base paani me completely ionise ho jata hai aur zyada matra me OH⁻ ions deta hai, use strong base kehte
Weak Base
Jo base paani me partially ionise hota hai aur kam matra me Hydroxide ions (OH⁻ ions) deta hai, use weak base kehte hain।
Properties of Bases
- Bases ka taste kadwa (bitter) hota hai।
- Bases touch karne par soapy / chikne lagte hain।
- Bases red litmus ko blue kar dete hain।
- Bases solution me electricity conduct karte hain।
- Bases kuch metals ke sath react karke Hydrogen gas banate hain।
Base + Metal → Salt + Hydrogen
2NaOH(aq) + Zn(s) → Na2ZnO2(aq) + H2(g)(Sodium Hydroxide) (Sodium zincate)
- Bases acids ke sath react karke salt or water banate hain
Base + Acid → Salt + Water
2NaOH(aq)+H2SO4(aq)→Na2SO4(aq)+ 2H2O
(Sodium Hydroxide) (Sodium sulphate)
- Bases non-metal oxides ke sath react karke salt aur water banate hain।
Non-metal oxide + Base → Salt + Water
Ca(OH)2(aq) + CO2(g) → CaCO3 + H2O
(Calcium Hydroxide) (Calcium carbonate)
Uses of Bases
- Sodium Hydroxide ka use soap, paper aur synthetic fibre (rayon) banane me hota hai.
- Calcium Hydroxide ka use bleaching powder banane me hota hai.
- Magnesium Hydroxide ko antacid ke roop me use kiya jata hai, jo stomach ke excess acid ko neutralise karke indigestion theek karta hai.
- Sodium carbonate ka use washing soda ke roop me aur hard water ko soft karne me hota hai.
Strength of Acid aur Base Solution
Acid aur base ki strength ko pH scale se measure kiya jata hai।
pH Scale
Acid ya base ki taakat (strength) ko pH scale se measure kiya jata hai।
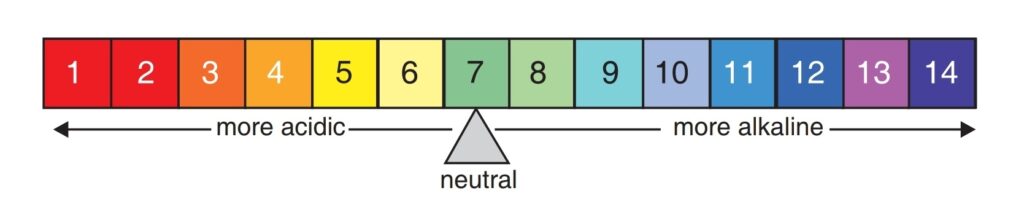
pH scale ki value 0 se 14 tak hoti hai।
1 Neutral substance ka pH bilkul 7 hota hai
Example: Pure water ka pH = 7
2 Acids ka pH 7 se kam hota hai
3 Bases ka pH 7 se zyada hota hai
Importance of pH in Everyday Life
Digestive System me pH
Hamare stomach me Hydrochloric acid (HCl) banta hai। Ye dilute HCl food ko digest karne me help karta hai bina stomach ko nuksan pahunchaye। Kabhi-kabhi kisi wajah se acid zyada ban jata hai, jisse indigestion, pain aur irritation hoti hai। Isse theek karne ke liye hum antacids (bases) lete hain.
Antacids ke example:
- Magnesium Hydroxide (Milk of Magnesia)
- Sodium Hydrogencarbonate (Baking soda)
pH changes as the cause of Tooth Decay (Daant ka kharab hona)
Jab hum sugar wali cheezein khate hain, to mouth ke bacteria sugar ko acid me badal dete hain। Isse mouth ka pH kam ho jata hai। Jab mouth ka pH 5.5 se neeche chala jata hai, tab tooth decay start ho jata hai। Kyunki tab acid daanton ke enamel ko attack karke corrode kar deta hai।
Soil pH and Plant Growth
Agar soil bahut zyada acidic ho jaye, to uska pH bhut km mho jata hai jiss se kheti achi nhi ho paati to usme quicklime, slaked lime ya chalk milaya jata hai taaki pH sahi ho sake
Animals aur Plants ka Self Defence (Chemical Warfare)
Jab honey-bee kisi ko sting karti hai, to skin me acidic liquid inject karti hai, jisse bahut pain aur irritation hoti hai to iss Is par baking soda (mild base) lagane se acid neutralise ho jata hai aur effect kam ho jata hai।
Ant sting me methanoic acid hota hai, jo burning pain deta hai to iss Is par baking soda (mild base) lagane se acid neutralise ho jata hai aur effect kam ho jata hai।
Salts
Salt ek compound hota hai jo acid ke hydrogen ki jagah metal aane se banta hai।
Note: Salt sirf sodium chloride ko hi nahi kehte, ye ek general name hai।
Salts acids aur bases ke reaction se bante hain।
Common Salt (Sodium Chloride – NaCl)
Laboratory me:
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
Is reaction se sodium chloride (common salt) banta hai।
Common Salt (Namak) ki Basics
- Sea-water se evaporation ke process se namak banta hai.
- Rock salt ko zameen ke neeche se coal ki tarah mine kiya jata hai.
Common Salt ke Uses
- Industrial Raw Material: Isse bahut saare chemicals bante hain jaise:
- Caustic Soda (Sodium Hydroxide)
- Washing Soda (Sodium Carbonate)
- Baking Soda (Sodium Hydrogencarbonate)
- Khane mein: Taste badhane ke liye. Hamari body mein nervous system aur muscles ki movement ke liye ye zaruri hai.
- Preservative: Achar (pickles), meat aur fish ko kharab hone se bachane ke liye.
- Soap Making: Sabun banane mein use hota hai.
- Ice Melting: Thande countries mein sadak par jami baraf pighlane ke liye.
Sodium Hydroxide (NaOH) – Caustic Soda
Jab hum namak ke paani (Brine) mein se bijli (electrolysis) pass karte hain, tab Sodium Hydroxide banta hai.
Equation:
2NaCl(aq) +2H2O(l) →2NaOH(aq) +Cl2(g) +H2(g)
Sodium Hydroxide ke Uses:
- Soap aur detergents banane mein.
- Artificial textile fibres (kapde) banane mein.
- Paper industry mein.
- Bauxite ore ko saaf karne mein (Aluminium nikalne ke liye).
- Metals ki de-greasing, oil refining aur dyes banane mein.
Chlorine (Cl2) ke Uses
- Bleaching powder banane mein.
- Drinking water aur swimming pools ko sterilise (keetano-mukt) karne mein.
- Hydrochloric acid (HCl) banane mein.
- PVC plastics, pesticides aur CFCs banane mein.
Hydrogen (H2) ke Uses
- Hydrogenation: Oil se solid fats (daalda) banane mein.
- HCl (Hydrochloric acid) banane mein.
- Fertilizers ke liye Ammonia banane mein.
- Methanol banane mein.
- Liquid Hydrogen ko rocket fuel ki tarah use kiya jata hai.
Hydrochloric Acid (HCl) ke Uses
- Cleaning: Iron sheets ko tin-plating ya galvanisation se pehle saaf karne ke liye.
- Medicines & Cosmetics: Iska use dawaiyon aur cosmetics banane mein hota hai.
- Industries: Textile (kapda), dyeing (rangne) aur tanning (chamda) industries mein kaam aata hai.
- Plastics: PVC (Polyvinyl Chloride) jaise plastics banane mein help karta hai.
Washing Soda (Na2CO3·10H2O)
Washing soda ko Namak (NaCl) se 3 steps mein banaya jata hai:
- Step 1: Namak, Ammonia aur CO2 ko milakar Baking Soda (NaHCO3) banaya jata hai.
NaCl+ NH3 +H2O +CO2→NaHCO3+NH4Cl
- Step 2: Baking soda ko garam karke Soda Ash (Na2CO3) milta hai.
2NaHCO3 + Heat → Na2CO3 + CO2 +H2O
(soda ash)
- Step 3: Soda ash mein paani milakar use Washing Soda (Crystallization) banaya jata hai.
Na2CO3 + 10H2O → Na2CO3.10H2O
sodium carbonate decahydrate Na2CO3.10H2O (washing soda)
- Ye ek chamakdar (transparent) crystal jaisa hota hai.
- Ye un kuch metal carbonates mein se hai jo paani mein ghul (dissolve) jate hain.
- Iska nature Alkaline hota hai, isliye ye Red Litmus ko Blue kar deta hai.
- Isme safai karne ki (detergent) property hoti hai.
Uses (Istemal):
- Gharon mein kapde dhone aur safai (cleansing agent) ke liye.
- Paani ki Permanent Hardness ko hatane ke liye.
- Glass, Soap aur Paper banane wali factory mein.
- Borax jaise sodium compounds banane mein.
Baking Soda (Sodium Hydrogencarbonate – NaHCO3)
Isko banane ka process wahi hai jo Washing Soda ke Step 1 mein tha:
Reaction:
NaCl+NH3 +H2O+CO2→NaHCO3 +NH4CI
NaHCO3→(Sodium Hydrogen carbonate)
Baking Soda (NaHCO3) ki Properties
- Ye white crystals hote hain jo paani mein thode kam ghulte (sparingly soluble) hain.
- Ye ek mild aur non-corrosive base hai (matlab isse jalan nahi hoti, isliye khane mein use hota hai).
- Action of Heat (Garam karne par): Jab hum ise khana pakate waqt garam karte hain, toh ye Carbon Dioxide (CO2) nikalta hai, jis se cake ya pakode phoolte hain.
2NaHCO3 + Heat → Na2CO3 + CO2 +H2O
NaHCO3→(Sodium Hydrogen Carbonate) Na2CO3→ (Sodium Carbonate)
Baking Soda ke Uses:
- Antacid: Pet ki acidity khatam karne ke liye dawa ki tarah use hota hai.
- Baking Powder: Cakes aur bread banane mein.
- Fire Extinguisher: Aag bujhane wale cylinders mein iska use hota hai.
Bleaching Powder (CaOCl2 – Calcium Oxychloride)
Kaise banta hai? Jab dry slaked lime par Chlorine gas pass ki jati hai.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Ca(OH)2→ (calcium Hydroxide) CaOCl2 → (calcium oxcychloride)
Properties:
- Ye ek white powder hai jis se Chlorine ki tez smell aati hai.
- Ye thande paani mein ghul jata hai.
- Acid ke saath react karke Chlorine gas chhodta hai.
Uses:
- Peene ke paani ko saaf (disinfect) karne ke liye.
- Chloroform banane mein.
- Wool (oon) ko sikadne (unshrinkable) se bachane ke liye.
- Industries mein oxidising agent ki tarah.
Plaster of Paris – POP (CaSO4.1/2H2O)
Isko Gypsum ko 1000C par garam karke banaya jata hai.
CaSO4.2H2O + Heat(100⁰c) → CaSO4.1/2 H2O + 3/2H2O
CaSO4.2H2O→Gypsum CaSO4.1/2 H2O → (Plaster of Paris)
Sabse Zaruri Baat (Setting Property):
POP mein jab paani milate hain, toh ye wapas se Gypsum ban jata hai aur bilkul patthar jaisa hard ho jata hai.
Note: Isiliye ise moisture-proof container (nami se door) mein rakhna chahiye, nahi toh ye box ke andar hi jam jayega.
POP ke Uses:
- Hospitals: Tooti hui haddiyon (fractured bones) ko sahi jagah par set karne ke liye.
- Decoration: Khilone aur ghar ki chhaton (ceilings) ka design banane mein.
- Fire-proofing: Aag se bachne wale material ke taur par.
- Labs: Apparatus ke air gaps ko seal karne ke liye takki wo air-tight ban sakein.
Download Free Acids, Bases and Salts Class 10 Handwritten Notes PDF
